
REIMAGINE VACCINES
Creating next generation vaccines
At Vaxeleron, we’re pioneering the future of RNA therapies.
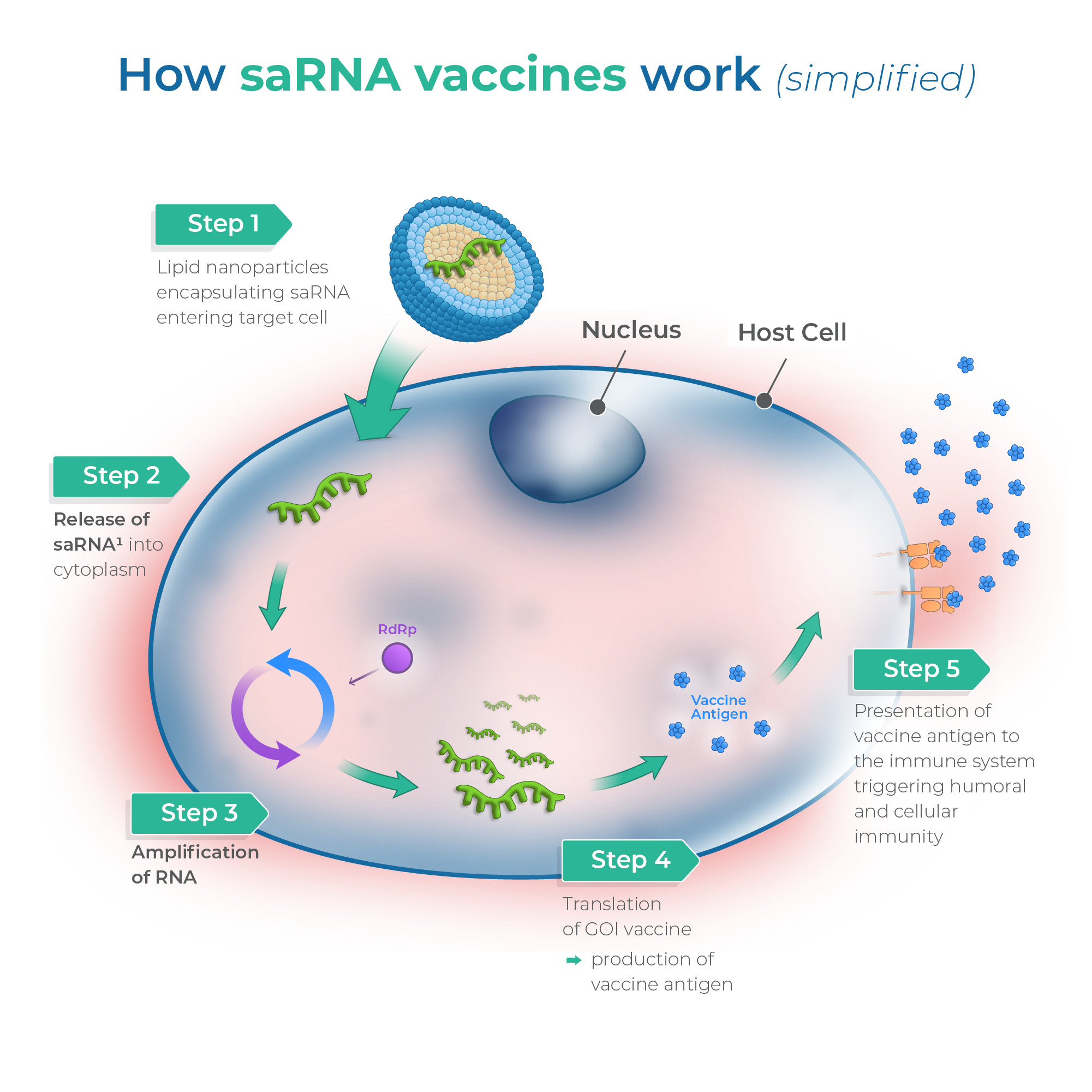
Our proprietary replicon platform is designed to deliver vaccines and therapeutics at very low doses thanks to the deployment of a self-amplifying system, resulting in administration of less formulated RNA.
Driven by purpose, Committed to Impact

Our Technology
Our proprietary replicon platform is designed to deliver RNA at significantly lower doses. This not only improves safety and efficacy but also lowers manufacturing costs, making next-generation vaccines more accessible and sustainable.
This is achieved by so called self-amplifying RNA, which allows for the body itself to make more of the administered vaccine. The benefit is that less RNA and formulation has to be administered to achieve an equally good protection from infectious agents.
OUR LEADERSHIP TEAM
EXPERIENCED LEADERSHIP
Our founders bring a wealth of expertise and a proven track record in vaccine research and development.

Randolph Seidler
CEO and Head of Business Development
Randolph is a co-founder and Vaxelerons CEO and has extensive R&D and portfolio leadership experience...
As Head of Global R&D and Regulatory Affairs for 10 years at Boehringer Ingelheim Animal Health (BIAH), he oversaw the global research, development and registration of multiple highly innovative drugs and vaccines. During this time BIAH brought multiple new molecular entities (vaccines and pharmaceuticals) to all major markets.
During his career he also held the position of Global Head of Business Development, Licensing and Alliance Management, where he oversaw the successful negotiation of several joint ventures, technology licenses and equity investments. Prior to this he held various international positions of growing responsibility within Boehringer Ingelheim’s human pharma R&D organization, including roles in cardiovascular diseases in the US and in Germany.
In addition to his role in Vaxeleron, Randolph supports several animal health startup companies through supervisory and advisory board positions. Randolph holds a degree in Veterinary Medicine and a Doctorate in physiology from the Ludwig-Maximilians-University in Munich, Germany and an Executive MBA from INSEAD in Fontainebleau, France.

Konrad Stadler
CSO
As Head of Global R&D and Regulatory Affairs for 10 years at Boehringer Ingelheim Animal Health (BIAH), he oversaw the global research, development and registration of multiple highly innovative drugs and vaccines. During this time BIAH brought multiple new molecular entities (vaccines and pharmaceuticals) to all major markets.
During his career he also held the position of Global Head of Business Development, Licensing and Alliance Management, where he oversaw the successful negotiation of several joint ventures, technology licenses and equity investments. Prior to this he held various international positions of growing responsibility within Boehringer Ingelheim’s human pharma R&D organization, including roles in cardiovascular diseases in the US and in Germany.
In addition to his role in Vaxeleron, Randolph supports several animal health startup companies through supervisory and advisory board positions. Randolph holds a degree in Veterinary Medicine and a Doctorate in physiology from the Ludwig-Maximilians-University in Munich, Germany and an Executive MBA from INSEAD in Fontainebleau, France.

Melanie Sno
MD and COO

Gianclaudio Antonelli
CFO
SCIENTIFIC ADVISORY BOARD

Albert Osterhaus
Professor Osterhaus has been Head of the Department of Viroscience at Erasmus MC Rotterdam until 2014, is currently Director of the Center of Infection Medicine and Zoonosis Research and Guest-Professor at the University of Veterinary Medicine...
Hannover. He has a long track record as a scientific researcher and Principal Investigator of numerous major scientific projects.
At Erasmus MC, Professor Osterhaus has run a diagnostic virology lab with more than 40 staff and a research virology lab with over 150 personnel. His research program follows a novel integrated “viroscience” concept, bringing together world-leading scientists in molecular virology, immunology, epidemiology, pathogenesis, and intervention studies on human and animal virus infections. Among the major accomplishments are the discovery of more than 70 new viruses of humans and animals (e.g., human metapneumovirus, coronaviruses, influenza viruses), elucidation of the pathogenesis of major human and animal virus infections, and development of novel intervention strategies. This has enabled health authorities like the WHO to effectively combat disease outbreaks like SARS and avian influenza. The spin-offs, Viroclinics -DDL, Vironovative and CR2O, together employing more than 450 people, allow effective testing and refining of diagnostic tools and other intervention strategies and illustrate additional societally relevant successes.
The international recognition of Professor Osterhaus is further highlighted by major prizes, guest lecture invitations, (co-)organizerships of international meetings and editorships of scientific journals. Professor Osterhaus has acted as mentor for more than 80 PhD students and holds several key patents. He is also the author of more than 1300 papers in peer-reviewed journals, together cited > 85,000 times, with an H index > 124.
Currently he also is Chair of the European Working Group on Influenza (ESWI). He organized numerous international scientific conferences on influenza and other emerging infections and received numerous prestigious awards.
He holds several senior editorships and is Chief Editor of One Health Outlook, a newly established journal of the Springer-Nature group.
He is member of the Dutch and German National Academies of Sciences, member of the Belgium Academia of Medicine, and Commander of the Order of the Dutch Lion.

Michael Houghton
Dr. Michael Houghton was the Canada Excellence Research Chair in Virology from 2010-2018 and is the Li Ka Shing Professor of Virology at the University of Alberta where he is also the Director of the Li Ka Shing Applied Virology Institute. He was jointly named the...
2020 Nobel Prize winner in Physiology or Medicine along with Harvey J. Alter and Charles M. Rice in recognition of the discovery of the hepatitis C (HCV) virus. He was knighted by Queen Elizabeth II in 2020 for services to medicine.
His research in the field of viral hepatitis has led to protection of the world’s blood supplies, and hepatitis C treatment to the point where the viral infection can now be cured in virtually all patients. He and colleagues have been working on a HCV vaccine first at Chiron/Novartis & subsequently at the University of Alberta, along with the US NIH, and have developed the only HCV vaccine candidate shown to be protective in animal models and which can neutralize the in vitro infectivity of most global strains of HCV.
Born in the United Kingdom, Houghton graduated from the University of East Anglia with a BSc in biological sciences in 1972, and subsequently completed his PhD in biochemistry at King’s College, University of London in 1977. Houghton joined G. D. Searle & Company in the UK studying human interferon gene regulation before moving to Chiron Corporation in California, USA in 1982 where together with Chiron colleagues Qui-Lim Choo and George Kuo, and Daniel W. Bradley from the Centers for Disease Control and Prevention, first discovered HCV in 1989. Houghton was co-author of a series of seminal studies published in 1989 and 1990 that identified hepatitis C antibodies in blood, particularly among patients at higher risk of contracting the disease, including those who had received blood transfusions. This work led to the development of a blood screening test in 1990, and widespread blood screening that began in 1992 with the development of more sensitive tests which has eliminated hepatitis C contamination of donated blood supplies in Canada and around the world. His group also identified key enzymes in the viral replication cycle that have been the target of successful drug development by international Pharma companies.

Geert Leroux-Roels
Geert Leroux-Roels received his MD from Ghent University in 1976. During his medical studies and specialization in internal medicine, he conducted doctoral research in clinical pathology and immunology. After obtaining board certification in internal medicine and...
a PhD in biomedical sciences, he completed postdoctoral research at the Scripps Research Institute in La Jolla, California, and at the Laboratory of Molecular Biology at Ghent University. In 1989, he was appointed professor of medicine and director of the Laboratory of Clinical Pathology.
For over 30 years, Dr. Leroux-Roels and his team have focused on the human immune response to HBV, HCV, HIV, malaria and influenza. They developed a small animal model (human liver in uPA-SCID mice), enabling in vivo studies of hepatotropic pathogens such as HBV, HCV, HEV, and Plasmodium falciparum (malaria).
Dr. Leroux-Roels founded the Center for Vaccinology (CEVAC) at Ghent University and Ghent University Hospital, directing the unit for three decades. Under his leadership, over 275 clinical vaccine trials were conducted, evaluating a wide array of candidate vaccines (HAV, HBV, [HAV+HBV], HSV, HPV, HIV, TB, malaria) and new adjuvant systems.
He is the author or co-author of 320 peer-reviewed articles and is an active member of several international societies and scientific advisory boards
